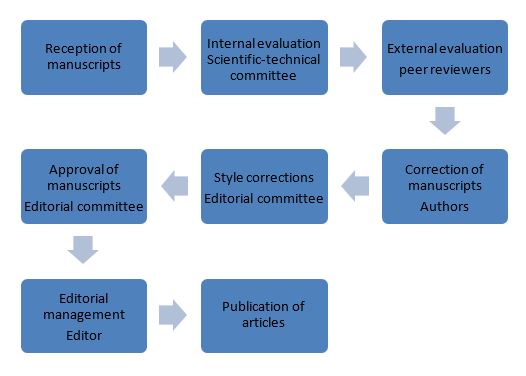
MANUSCRIPT EVALUATION PROCESS
1. RECEPTION OF PAPERS
All manuscripts must be sent to the e-mail address revistaiics@fumc.edu.co along with the following documents: Letter of presentation, manuscript registration and check format, completely filled out. At the time of receipt of the information, the editor will be responsible for sending a notification of submitted documentation.
The editorial committee receives and evaluates the articles that are submitted to the journal.In the first instance, an internal evaluation is carried out, where the editorial committee verifies if the manuscript has the basic requirements set forth in the notes to the authors.Subsequently, a member of the scientific – technical committee, which may belong to the university community or be a guest to participate in the process during one or more editions, evaluates the relevance and quality of the document, through the completion of the » Editorial Committee Evaluation Form». This first concept will be the starting point to determine if the manuscript continues in the evaluation process or if it should be rejected, in which case, the editor proceeds to notify the authors of the decision of the committee with its adequate justification.
2. EXTERNAL PEER EVALUATION
Manuscripts approved in the first process are evaluated by external peers. At this point, the editorial committee of the journal makes the decision to send the manuscript to a member of the national or international scientific community, considered an expert on the specific subject of the manuscript. This person is formally invited to participate in the process through an official communication or email. In case of accepting the invitation, the manuscript is sent without any information that allows the peer to know the identity of the authors (blind evaluation), the «Peer Evaluation Form» and a delivery date. Each evaluator has an approximate period of 15 days for the delivery of their evaluation.
3. EXTERNAL PEER CONCEPT
Once the evaluations have been delivered by the external peers to the journal. The committee proceeds to send the concept of the evaluations to the authors with the respective corrections. In case the concept is publishable or publishable with corrections, the authors must complete the «Format for Confrontation of Evaluations» and make the corresponding corrections. Both documents must be sent again to journal via email.
4. AUTHOR’S CORRECTIONS
After receiving the corrections of the authors, the editorial committee is responsible of verifying that the concepts issued by the peer reviewers correspond with the corrections made to the manuscript and the publication is proceeded through the completion of the authorization for publication form
5. EDITORIAL MANAGEMENT
The editorial committee is responsible for making a final revision of form and style, to complete the editorial process and proceed to the publication of the article.
In compliance with the regulations of Colciencias for scientific publications in Colombia, the Journal has OJS 3.0 system (Open Journal Systems 3.0) for the Document Management and the editorial process.
EDITORIAL PROCESS FLOW DIAGRAM